







IPCS |
International Programme on Chemical Safety |
CHEMICAL SAFETY TRAINING MODULES
PART I: INTRODUCTION TO SAFETY IN THE USE OF CHEMICALS
Chemicals have become a part of our life, sustaining many of our activities, preventing and controlling diseases, and increasing agricultural productivity. However, one can not ignore that these chemicals may, especially if not properly used, endanger our health and poison our environment.
An estimation of one thousand new chemicals enter the market every year, and about 100000 chemical substances are used on a global scale. These chemicals are mostly found as mixtures in commercial products. One to two million such products or trade names are available.
More substances and rising production mean more storage, transport, handling, use and disposal of chemicals. The whole lifecycle of a chemical should be considered when assessing its dangers and benefits.
Most chemical accidents have a limited effect. Occasionally there is a disaster like the one in Bhopal, India, in 1984, with thousands of deaths and many people permanently disabled.
Not only the worker handling chemicals is at risk. We may be exposed to chemical risks in our homes through misuse or by accidents. The environment may be affected, chemicals may pollute the air we breathe, the water we drink, and the food we eat. They may have entered into forests and lakes, destroying wildlife and changing the ecosystems.
Chemicals are not all of equal concern. The assessment of health risks of chemical substances is a continuous process where information of the chemical hazards and exposure patterns are made available through a variety of sources.
2. How can workplace chemicals enter our body
| No chemical substance can cause adverse effects without first
entering the body or coming to contact with it. There are four main ways, that is routes of exposure, for chemical substances to enter the human body:
|
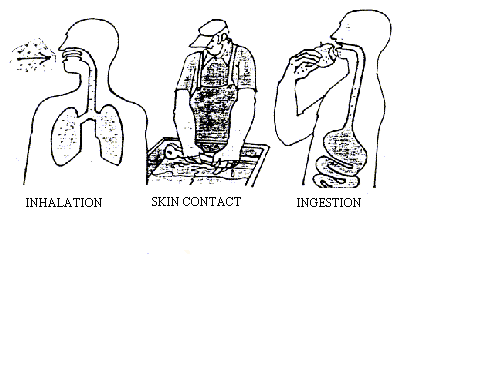 |
Most chemicals used at the place of work may be dispersed into the air to form dust, mist, fumes, gas or vapour and can then be inhaled. In this way also workers who are not actually handling them but stay within the reach can be exposed to a mixture of chemicals from various sources. Handling chemical substances without proper precautions exposes the worker to the risk of absorbing harmful amounts of chemical through the skin. This usually takes place when the chemical is handled in liquid form. Dust may also be absorbed through the skin if it is wetted by, for instance, sweat. The capacity of different chemical substances to penetrate the skin varies considerably. Some substances pass through it without creating any feeling. Skin absorption is, after inhalation, the second most common route through which occupational exposure may take place. |

|
The protective external layer of skin may be softened (by toluene, dilute washing soda solution) thus permitting other chemicals to enter readily to the bloodstream (such as aniline, phenol, benzene).
Eyes may also absorb chemical substances, either from splashes or from vapours.
Dangerous chemicals can enter the body through ingestion as gases, dusts, vapours, fumes, liquids or solids. Inhaled dust may be swallowed, and food or cigarettes may be contaminated by dirty hands.
Eating, drinking and smoking should be prohibited at the place of work where dangerous chemicals are used.
Whatever the route of entry, chemicals can reach the blood stream and be distributed all over the body. In this way damage can be caused at the site of entry as well as to organs distant from the exposed area.
The harmful effects of chemical substances depend on the toxicity and the exposure to that chemical. Toxicity is a property of the chemical substance, while the exposure depends on the way the chemical is used.
The level of exposure depends on the concentration of the hazardous chemical and on the period of contact time.
Many substances do not give any warning by odour, even though they may be present at dangerous concentrations in the workplace air.
3.1 Acute effects - Chronic effects
The effects may be acute: after a short exposure an immediate effect may be experienced. Chronic effects usually require repeated exposure and a delay is observed between the first exposure and appearance of adverse health effects.
A substance may have acute and chronic effects. Both acute and chronic conditions can result in permanent injury.
Injury from exposure to a chemical substance can be temporary, i.e. reversible. It will disappear when exposure to that chemical stops.
Exposure to solvents may cause contact dermatitis, headache or nausea. These effects could be both acute and temporary. Solvents can also cause chronic effects and result in an irreversible, permanent injury to the nervous system.
3.2 Local effects - Systemic effects
Hazardous substances may cause local effects. Acute local effects may include corrosive injuries from acids and bases or lung injuries from inhaled gases such as ozone, phosgene and nitrogen oxides.
Many other gases cause adverse effects only after they have been inhaled repeatedly over a long time period. Low concentrations of a gas may also act in this way. A persistent irritation of the respiratory system can arise from exposure to gases such as sulphur oxides, hydrogen fluoride and hydrogen chloride.
Once the hazardous substance has entered the blood circulation, it may be distributed to all parts of the body. It will reach the liver, which is the most important detoxication organ of the body. The liver attempts to convert the toxic agents to a less toxic ones or to the ones useful to the body. This process is called metabolism. Some substances such as alcohol and carbon tetrachloride can damage the liver.
The body excretes unwanted chemicals. The kidneys filter them from blood circulation, which is the main way that the body excretes poisons, but in doing this, the kidneys can be damaged by toxic substances, such as carbon tetrachloride, ethylene glycol and carbon disulphide. Cadmium causes permanent damages to kidneys.
Other means of excretion are via faeces, sweat and through lung exhalation.
The nervous system is sensitive to chemicals. The adverse effects may be on the central nervous system or on the nerves that transport impulses to other parts of the body.
Organic solvents are commonly used at work and are known to be able to affect the nervous system. An example is tetraethyl lead, a gasoline additive, which causes skin effects at the contact site. Then it is absorbed and transported into the body causing typical effects on the central nervous system and on other organs.
Many other substances may behave in the same way, such as carbon disulphide, mercury, lead, manganese and arsenic.
The degree of the toxic effect is not the same in all organs. Usually there are one or two organs which are most affected. These are referred to as target organs of toxicity of the particular substance. The central nervous system is the target organ of toxicity most frequently involved in systemic effects. The blood circulation system, liver, kidneys, lungs and skin follow in frequency. Muscle and bones are the target organs for a few substances. The male and female reproduction systems are vulnerable to many substances.
- Skin is the largest organ in the human body, 1.5-2 m2 in area. It provides a protective cover to the body but can fail if the load is overwhelming. A number of substances can penetrate healthy intact skin and enter the blood circulation. Phenol is a substance that may even result in death after exposure and penetration through the skin.
The vast majority of work-related skin diseases are contact eczema, irritation and inflammation of the skin. This condition can be either a non-allergic or allergic reaction to exposure to chemical substances. Several colorants and dyes, metals such as nickel and its salts, chromium and cobalt salts and organomercuric compounds, monomers of a number of acrylates and rubber additives are examples of common contact sensitizers. In practice also circumstances, such as humidity and heat, influence the formation chemical skin injury.
- The lung is the major route through which toxic substances enter the body in the workplace. It is also the first organ to be affected by dusts, metal fumes, solvent vapours and corrosive gases. Allergic reactions may be caused by substances such as cotton dust, TDI (toluene diisocyanate, used in the manufacture of polyurethane plastics), and MIC (methyl isocyanate, used in production of carbaryl insecticide). In a disastrous chemical accident in Bhopal, India, in 1984, more than 2000 people died from exposure to MIC.
Allergic reactions may result also from exposure to bacteria or fungi: this is the case in allergies from handling stocked hay (`farmer's lung') or dried sugar cane.
When dust particles of a certain size of some substances are inhaled the lungs are unable to remove them. The particles become embedded in the lungs causing a condition called pneumoconiosis. Pneumoconiosis is a specific problem for workers exposed to the dust of silica (quartz) and asbestos, and is the most common non-malignant occupational lung disease throughout the world.
Other substances, such as formaldehyde, sulphur dioxide, nitrogen oxides and acid mists, may cause irritation and reduce the breathing capacity.
- The nervous system is sensitive to the hazardous effects of organic solvents. Some metals affect the nervous system, especially heavy metals such as lead, mercury and manganese. Organophosphate insecticides such as malathion and parathion interfere severely with information transmission (chemical neurotransmitter function) in the nervous system, leading to weakness, paralysis and sometimes death.
- The blood circulation is a target for the adverse effects of solvents. Blood cells are mainly produced in the bone marrow. Benzene affects the bone marrow; the first sign is mutation in the blood cells called lymphocytes. To study mutation, lymphocytes are cultured in the laboratory to observe specific types of cellular changes.
Lead, in the form of the metal or its compounds, is another classic example of a chemical that may cause blood problems. Lead in the blood may inhibit certain enzyme activities involved in the production of hemoglobin in red blood cells. Chronic lead poisoning may result in a reduced ability of the blood to distribute oxygen throughout the body, a condition known as anaemia.
- The liver, the largest of the internal organs of the body, has several important functions. It is a purification plant which breaks down unwanted substances in the blood. The liver has a considerable reserve capacity; symptoms of liver disorder appear only in serious diseases. Solvents such as carbon tetrachloride, chloroform and vinyl chloride, as well as alcohol, are hazardous to the liver.
- The kidneys are part of the body's urinary system. They have the task of excreting waste products that the blood has transported from various organs of the body, of keeping the fluids in balance and of ensuring that they contain an adequate blend of necessary salts. They also maintain the acidity of the blood at a constant level.
Solvents may irritate and impair kidney function. The most hazardous to the kidneys is carbon tetrachloride. Turpentine in large quantities is also harmful to the kidneys: `painter's kidney' is a known condition related to occupational exposure. Other well-known kidney-damaging substances are lead and cadmium.
| An allergic reaction, or sensitization as it is also called,
may appear after repeated contact to a substance. Once the sensitization has bee produced,
even very low doses can provoke a reaction. Numerous allergies are exist, varying from
minor skin irritation to very severe or even fatal reactions. The pattern of sensitization varies according to the species. In humans, the skin and the eyes are the most common areas of allergic response, whereas, for example, in the guinea pigs reactions are more common in the respiratory system. |
 |
| The effect of simultaneous exposure to two or more substances
may differ from a simple additive effect (1+1=2). Organophosphate pesticides, such as
dialiphos, naled and parathion, are examples of chemicals where the combined effect is the
sum of the effects observed when the chemicals act individually. The effect can be more than the sum of the individual effects of two chemicals (e.g., 1+1=4). An example of an increase in risk is with asbestos fibres and cigarette smoking. They act together: the risk of developing lung cancer after exposure to asbestos fibres is forty times greater for a smoker than for a non-smoker. Another pair of the chemicals where the combined risk is greater than a mere additive effect are the solvents trichloroethylene and styrene. |
 |
The adverse effects of two substances may counteract one another (1+1=0). This effect is used to find an antidote to a poison.
In some cases, a substance may not cause harm on its own but may make the effect of another chemical much worse (0+1=3). For example, two commonly used solvents isopropanol and carbon tetrachloride have this kind of joint effect. Isopropanol, at concentrations which are not harmful to the liver, increases the liver damage caused by carbon tetrachloride.
In some cases, when the exposure to a substance is repeated the body may decrease its sensitivity to the substance, i.e. it increases its tolerance to it.
Our body has a considerable capacity to excrete, to render dangerous substances harmless, and to protect us. However, our defense system can be overloaded by repeated heavy exposure so that it no longer fulfills its function. The body may store the harmful substance which may consequently result in health problems.
Lead is an example of a substance for which removal from the body takes a long time. Cadmium is an example of a substance that will stay in the body once it has entered there.
Toxicology is the science of adverse effects of chemical substances on living organisms.
Living organisms include the algae in the sea, animals and people, all flora and fauna.
There are no safe substances, all chemicals can be poisonous and cause injury or death. But they can be used safely: the effect depends on the dose and exposure. It is possible by limiting these to handle and benefit from the properties of chemical substances in an `acceptably safe' way.
Toxicological studies aim to assess the adverse effects related to different doses in order to find this `acceptably safe' level.
The work is carried out in two phases: first by collecting data on the properties of chemicals, results of studies and accidental misuse of chemicals, and then by predicting the effects of chemicals in different situations.
To make relevant predictions there must be information available on:
This information is obtained from laboratory tests with cells, bacteria, animals, epidemiological studies and from accidents involving the substance.
Large amounts of toxicological information has been collected into data bases and data banks.
In toxicological animal experiments the routes of exposure may be:
For many substances the greatest effects and the most rapid responses occurs when the substance is inserted directly into the blood circulation.
4.2 LD50 and LC50; comparison of acute toxicity
LD50 is the abbreviation used for the dose which kills 50% of the test population.
LC50 is the abbreviation used for the exposure concentration of a toxic substance lethal to half of the test animals.
LD50 is expressed in milligrams per kilogram of body weight of the test animal (which must be mentioned).
LC50 is expressed in millilitres per kilogram of body weight of the test animal (which must be mentioned), exposed to the substance by inhalation during a specified period.
For different substances the doses needed to produce an adverse effect varies widely. LD50 values are used to compare acute toxicity.
The assessment of the effects is tested in laboratories using animals, mainly rats, mice and rabbits. The test substance or preparation may be applied to the animal orally, under the skin, by inhalation, into the abdomen or into the vein. LD50 and LC50 are the parameters used to quantify the results of different tests so that they may be compared.
Classification may be based on the LD50 and LC50 values (see Annex 7 in Identification, Classification and Labelling of Chemicals' and `Major Hazard Chemicals).
The following list describes the variation in LD50 values measured in ingestion studies of some substances on the rat:
| Substance | LD50 (mg/kg, oral, rat) |
| Vitamin C | 11 900 |
| Ethyl alcohol (`alcohol') | 7 060 |
| Citric acid | 5 040 |
| Sodium chloride (table salt) | 3 000 |
| Ferrous sulphate | 320 |
| Dieldrin | 38 |
| Parathion | 2 |
| Dioxin (contaminant in herbicide) | 0.02 |
It is important to mention the species on which the test was conducted because the numerical values of LD50 and LC50 depend on several factors, such as the biological system or animal, strain, sex, age and diet. The LD50 of DDT insecticide administered orally is 87 mg/kg of body weight for a rat but 150 mg/kg of body weight for a dog. The LD50 for dioxin is 0.02 mg/kg of body weight for a rat and 0.001 mg/kg of body weight for a dog, i.e. the rat is twenty times more tolerant to dioxin than the dog.
The assessment of how a human system would react is not straight forward estimation from the animal tests. However, the animal tests give an idea of the level of the toxic effects.
In order to control toxic effects, there is a need to set priorities, goals and strategies. In places of work one way is to set limit values to guide the users.
Occupational limit values are based on the best available information from industrial experience, from experimental laboratory studies and from accidents. They are informed and negotiated compromises, not fixed safety standards.
There are different kinds of limit values.
The TLVs (Threshold Limit Values) are published by the American Conference of Governmental Industrial Hygienists (ACGIH) and concern the airborne concentrations of hazardous substances. They set a limit concentration below which it is believed that nearly all workers can be repeatedly exposed day after day without adverse effect. The TLVs are regularly reviewed and corrected when new information becomes available.
TLV-TWA (Threshold Limit Value - Time Weighted Average) is a time-weighted average concentration for an eight hour working day or 40 hours a week to which nearly all workers may be repeatedly exposed without adverse effect.
TLV-STEL (Threshold Limit Value - Short Term Exposure Limit) is the concentration to which workers may be exposed for a short time (usually 15 minutes) without suffering from irritation, long-term or irreversible tissue damage or impairment likely to increase accidental injury, affect self-rescue or reduce work efficiency. Daily TLV-TWA values should not be exceeded.
TLV-C (Threshold Limit Value - Ceiling) is a concentration that should not be exceeded at all during work exposure.
5. Common chemical groups that cause health risks
| Dust may be just a nuisance, and the danger depends on the
type of material in the dust, and on the amount and the size of the particles. The smaller
the particle is the deeper it will penetrate into the lungs with the inhaled air, thereby
passing the defensive systems of the lungs. This type of dust is invisible to the eye and
identified using microscope technique. Such dust can accumulate in the lungs over a long
period of time and cause a lung disease called pneumoconiosis. Dusts containing
crystalline silica or asbestos most frequently can cause this condition. Sand and many
types of stone contain crystalline silica, as do many ores, concrete, ceramics and
diatomite. Processing of these materials creates dust with result of silica accumulating
in the lungs. This may lead after years to a incurable lung disease, even though the
exposure has been stopped years before. Asbestos is a natural mineral fibre which is very resistant to fire and to many chemicals. Asbestos fibres are very strong and thin. Asbestos exists in various forms and names: chrysotile, crocidolite, amosite, antophyllite, actinolite and tremolite asbestos. Chrysotile is used in isolating materials, protective carpets and clothes. The asbestos dust particles penetrates the lungs destroying the lung tissue. This condition is called asbestosis. Asbestos can also cause lung cancer. The risk of cancer is many times higher if the asbestos exposure is combined with smoking. Many countries have restricted or banned the use of asbestos. Exposure to metal fumes can cause damage to the body. `Metal fume fever' is a known health effect when metal fumes, often containing zinc, are inhaled. It usually appears on the day following that of the exposure. Gases do not necessarily have a warning odour at a dangerous concentration. The odour may be apparent only at very high concentration in the air. Gases may have an irritating effect, or they may enter the blood circulation and cause internal damage. Sulphur oxides, nitrogen oxides, chlorine and ammonia are toxic gases that are corrosive and irritating to the respiratory system. They are widely used in industry. Phosgene is formed when solvents containing chlorine, such as 1,1,1-trichloroethane and trichloroethylene, come into contact with hot surfaces or flames. Phosgene can be deadly poisonous even before the odour is detected. Carbon monoxide is a toxic, odourless, colourless gas which is formed by the incomplete burning of materials of organic origin. It may enter the blood circulation. Some gases can pass through the skin, for example, hydrogen cyanide. |
|
Most industrial solvents are liquid organic chemicals. They are used because of their ability to dissolve other substances, particularly fat and grease, which are insoluble in water. Many of them evaporate rapidly at ambient temperatures. They are often flammable and may ignite by heat from smoking, welding or static electricity. Vapours move with air currents and can ignite even by a distant heat source.
Inhalation is the most common way for solvents to enter the body, but some of them penetrate intact healthy skin. Once in the blood stream a solvent can be transported to different organs, such as the brain and liver.
Solvents have different effects on humans, depending on their evaporation rate and their solubility in water. The risks of health effects depend on the period of exposure and the concentration of the solvent in the inhaled air.
Many solvents have a narcotic effect; they may cause dizziness, headache, reduced comprehension or tiredness. They may also irritate the eyes and the respiratory tract. Frequent skin contact defats the protective layer of the skin causing irritation. Some solvents are very hazardous to the liver, kidneys, bone marrow or nervous system. Benzene, carbon tetrachloride and carbon disulphide belong to the category of solvents which should be substituted to less dangerous ones.
Metals can enter the body in the form of dust and fumes (in grinding or welding) or even through the skin.
Lead is used in various industries: battery, glass and mining industries, cable manufacturing, foundries and in printing works. Steel constructions are protected with anti-corrosive paint containing lead, which may be released during welding operations, for example, on ships.
Mercury is present in many pesticides and pickling baths. Mercury vapours are inhaled, as this liquid metal evaporates readily at room temperatures. In the environment, it may accumulate in fish. Mercury poisoning has serious effects on the nervous system.
Nickel is present with other metals in various alloys. Nickel and its compounds are known to be sensitizers. Once a person has had an allergic reaction to nickel, the reaction reoccurs following the contact with very small amounts of nickel used in products such as leather, cement, or door handles. Some compounds of nickel can cause cancer.
Chromium compounds, particularly chromates and bichromates, are widely used in industry. Cement contains small amounts of chromium compounds. These compounds can cause allergy and even lung cancer. Unlike cobalt and nickel, pure metallic chromium does not cause allergy.
Chromium compounds may cause birth defects if mothers are exposed to these compounds during pregnancy.
Arsenic compounds are used in pesticides, insecticides and in some colouring materials. Chronic arsenic poisoning can start with irritation to the respiratory system, inflammation of the eyes, or skin problems, followed by damage in nervous system. Arsenic and its compounds can cause cancer.
Strong acids and bases are mostly used as water solutions. They are corrosive to human tissue. Working with acids or bases can give rise to mists which have the same corrosive properties as the solutions.
When acids and bases are mixed with each other the phenomena of neutralization occurs, usually with strong production of heat. The heat production has particularly serious effects when water is added to concentrated sulphuric acid: the heat will splash the highly corrosive liquid up.
Some acids are explosive when in contact with organic material, such as sawdust.
Serious damage can result when treating metal pieces in acid bath. The bath may contain a mixture of acids and release flammable hydrogen gas, as well as acid mist, when a piece of metal is placed in it.
Phosphoric acid is used to treat metals. When in contact with hot surfaces, phosphoric acid can give off poisonous gases.
Ammonia, sodium and potassium hydroxides are commonly used bases. They are corrosive to human tissue in such a way that a certain period of time is required before the corrosive feeling is sensed. Bases penetrate the skin and cause deep sores. They are difficult to wash away. Dilute water solutions are irritating.
Sodium and potassium hydroxides are used, for example, in hot degreasing baths for cleaning metals.
Pesticides are intended to destroy or control pests of all kind. They are used in industry, for example, to impregnate wood, and in agriculture to control insects, weed, fungi, and rats. Different types of pesticide compounds and their mixtures are available by various trade names.
Some countries apply restrictions in using certain pesticides, and the use of some of them is banned because of their serious adverse effects. In Europe, the list of banned pesticides includes compounds such as inorganic mercury compounds, camphechlor, chlordane, dieldrin, DDT, HCH (lindane), heptachlor, hexachlorobenzene, and nitrofen.
Pesticides may be classified to groups according to the hazard they pose to human health and the environment. WHO recommended classification is mainly based on LD50 values and is divided to following categories:
List of technical products classified EXTREMELY HAZARDOUS
Examples of the compounds of this class are Aldicarb, Chlormephos, Parathion
List of technical products classified HIGHLY HAZARDOUS
Examples of the compounds of this class are Aldrin, Antu, Warfarin
List of technical products classified MODERATELY HAZARDOUS
Examples of the compounds of this class are Cyanofenphos, Cypermethrin, Sulfallate
List of technical products classified SLIGHTLY HAZARDOUS
Examples of the compounds of this class are Allethrin, Kelthane, Malathion
6. How to minimize the risks caused by chemicals
Improving safe use of chemicals can be achieved at different levels.
A Safety Committee should be formed with the task of working regularly with safety issues. It could start to work with following:
Organizational measures
Technical measures to control the hazard
| Technical measures can be used to prevent chemical hazards at
source. By technical means it is possible to reduce the exposure of the worker. Substitution An effective control method for any hazardous chemical is substitution: a hazardous chemical is replaced with a less hazardous one. This is especially important when the chemicals in question can cause cancer, damage to the reproductive functions or create allergic reactions. Choosing a safer process or changing an old and hazardous process to a less dangerous one effectively reduces the risks. |
 |
An example of safer choice is to have pellets or paste instead of powdered substances which readily produce high levels of dangerous dusts. Water-based paints and adhesives are available to replace harmful products containing solvents.
All possible information should be made available when considering the change of a substance or the whole process so that the new choice does not create unexpected new dangers.
Closed system
If hazardous chemicals can not be replaced by less dangerous ones, exposure must be prevented by protecting the worker. Enclosing the hazardous process or chemical is an effective method.
One example is to use sealed pipes to transfer solvents and other liquids instead of pouring them in the open air. Vapours and gases caused by spray painting or produced in pickling or hardening baths in the metal industry should be controlled, ventilated and not allowed to enter the workplace air.
Local exhaust ventilation
It is not always possible to enclose all dangerous operations. A properly designed local exhaust ventilation is the second choice in order to remove the contaminants at the source. A local exhaust ventilation system consists of a hood, ducts or pipes, a system to collect and separate the pollutants from the clean air, and an efficient fan to create enough suction force.
The hazardous gases, fumes and dust can be collected from the vented air. They should not go untreated, straight out, to pollute the surroundings of the factory and the environment.
Attention should be paid to the clean air inflow which replaces the exhaust. Inspection, proper maintenance, regular cleaning and changing of filters are essential to protect the workers against hazardous contaminants.
General ventilation
Where it is difficult or impossible to prevent hazardous chemicals, fumes, dusts, mists or particles from entering the workplace air at the source, a general dilution ventilation can be installed. This should be designed to meet the needs of the specific work process and workplace. At its best it should consist of an inflow of clean air and an outflow of exhaust forced by fans at right places. It can also be used with other preventive measures.
When working with dangerous chemicals, proper housekeeping is essential. Storage areas must be well organized and kept in order. The transport of chemicals within the industrial premises should be planned and the transport routes kept clear. Maintenance of premises and equipment should also be planned. These tasks should be dedicated to appointed persons/work groups/departments. Workers using the equipment should know the person responsible for repairing faulty equipment. Monitoring the efficiency of housekeeping and inspections should be carried out regularly; this should involve the workers themselves, who are experts in their own work.
| A `Code of Practice' has been developed by the International Labour Organisation, and some countries have applied these principles for organizing hazard control. This may involve the following activities at the shop-floor level: Safety Committee could initiate to |  |
| Planning and proper maintaining of storage areas is very
relevant for users of chemicals in order to avoid material losses, accidents and
disasters. Hazardous substances can leak, cause a fire or give off dangerous fumes and vapours. When two substances come into contact with one another, they may react violently. The reaction products may be much more dangerous than the original chemicals. Special attention should be paid to incompatible substances, suitable location of products within the storage area and proper arrangements and climatic conditions. For example, cylinders should be fixed with chains to upright position; the acids in the area or cupboard meant only for them. The acid fumes or splashes should never reach the area where cylinders are kept. Written instructions of storage practices should be provided, and Chemical Safety Data Sheets of dangerous substances kept in the stock, and should be available in the storage area. |

|
6.4 Chemicals in the environment/waste
Chemicals react in the same characteristic ways whether they are wastes or are used in a production process. The hazards are also the same.
The environment has a certain capacity to biodegrade toxic substances. However, some substances are resistant to decomposing processes. The adverse effects increase with the concentration of these substances and their accumulation in food chains.
In the natural environment, large numbers of potentially toxic substances are present. In some cases, when the substance is on its own it would cause no harm but it may interact with other toxic substances or under specific conditions it may be concentrated or transformed to a more dangerous compound. An example of an air pollution reaction is the production of photochemical smog in large cities.
Chemicals may add the adverse effects: chlorinated hydrocarbons such as DDT and dieldrin have similar chemical and biological effects. When present together they lead to more serious effects than when acting separately.
Where chemicals are used, the enterprise should plan the whole life cycle of the chemical, also the disposal of the chemical. The planning should include labelling, collecting and handling of wastes. Some countries have introduced legislation and provide detailed advice on how to treat dangerous chemical waste.
From the shop-floor, where the chemicals are actually used, up to the management, which should plan the whole, safe `lifecycle' for every substance, as well as cooperation with and within authorities is needed to use chemicals to benefit and to minimize the hazards from the use.
Many accidents have based on a false belief that everyone is aware of the situation.
Discuss in the work place the ideas arising when asking the following questions:
CHECKLIST TO SAFETY MANAGEMENT
WHAT IS A HAZARDOUS CHEMICAL?
A health risk
Fire and explosion hazard
Dangerous for the Environment
WHAT ARE YOU EXPOSED TO?
The actual hazard can be a poisonous chemical in form of dust, fume, gas, vapour, mist, liquid, solid, the danger can be oxygen deficiency, hot splashes,...
THE AMOUNT OF THE HAZARDOUS CHEMICAL, WHAT IS THE CONCENTRATION?
Respirators of different type have different levels of efficiency. There are agreed acceptable levels of concentration for many hazardous chemicals "Exposure Limits".
HOW LONG ARE YOU GOING TO BE EXPOSED?
You need to know the time for how long the cartridge/filter of the respirator can be worn before it has to be changed. The time gloves withstand certain chemicals is limited.
ANY OTHER CHEMICALS PRESENT?
Protective equipment is often meant for certain chemicals only: acid vapour filter can not hold back ammonia vapours. Goggles do not stop poisonous solvent penetrating through skin.
PREVENTIVE MEASURES
Substitution
Engineering control
Safe working procedures - Codes of Practice
Reducing exposure
Personal protective equipment
Monitoring
Hazard communication
Training
EXPOSURE BAN FOR PREGNANT EMPLOYEES
Work in pressurized enclosures and underwater diving
Toxoplasma virus
Rubella virus
Lead and lead derivatives
Underground mining work
EXPOSURE BAN FOR BREASTFEEDING MOTHERS
Lead and lead compounds
Underground mining work
CODE OF PRACTICE AT SHOP-FLOOR
Inventory list of all chemicals used at place of work
Summary of the health effect of those chemicals
Routes of exposure to those chemicals
Outline of equipment needed to do the work safely and correctly
Instructions of procedures for operating equipment correctly, including information on measures in case of irregularities, emergency situations etc.
Description of any personal protective equipment which may be necessary with detailed instructions for use and maintenance
Monitoring plan, details how often, in which way and what environmental and medical monitoring should be performed
Emergency plan and training
PERSONAL PROTECTIVE EQUIPMENT
Correct equipment
Training and follow-up training for workers required to use the equipment, are needed
Maintenance programme including regular cleaning,inspection and replacement of items, such as gloves and respiratory filters
Tests to ensure the good condition of equipment
It is especially important to check the face masks and respirators
Responsible person to take care of the equipment
Individual set of equipment to each worker and a secure, clean place to store the equipment
SUBSTANCES WHICH MAY PRODUCE OCCUPATIONAL LUNG DISEASES
The list contains examples of chemical substances or processes were workers may be exposed to the concentration level that cause various adverse effects in respiratory system.
Occupational exposure: by inhalation
| Substance | Source |
| Asbestos | Mining, construction, manufacture of materials containing asbestos |
| Aluminium | Manufacture of fireworks, ceramics, paints, electrical goods, abrasives, smelting |
| Ammonia | Ammonia production, manufacture of chemicals, fertilizers, explosives |
| Arsenic | Pesticides, pigments, alloys |
| Beryllium | Ore extraction, some alloys, ceramics |
| Cadmium | Electrical equipment, alloys, pigments, welding, smelting |
| Chlorine | Bleaching, manufacture of paper and pulp, water purification, chlorinated chemicals and pesticides |
| Chromium | Welding, metal treatment baths, paint pigments, production of chromium compounds |
| Coke oven emission | Coke production |
| Hydrogen fluoride | Solvent, plastics, films, manufacture of chemicals |
| Kaolin | Pottery production |
| Manganese | Various sources in metal and chemical industry |
| Nickel | Ore extraction, smelting, electroplating, fossil fuel |
| Nitrogen oxides | Welding, silo filling, explosives, exhaust gases |
| Organic dusts (cotton, hemp, etc.) | |
| Ozone | Welding, bleaching, water purification |
| Phosgene | Pesticide and plastic production, accidental releases from chlorine containing chemicals (for example, by heating) |
| Perchloroethylene | Dry cleaning, metal degreasing, grain fumigating |
| Silica | Mining, stone cutting, construction, farming, quarrying |
| Sulphur dioxide | Manufacture of chemicals, fumigation, bleaching, refrigeration |
| Talc | Rubber and cosmetics industry |
| Tin | Mining and processing of tin |
| Toluene 2,4-diisocyanate | Manufacture of plastics |
| Xylene | Important solvent, manufacture of resins, adhesives, paints and other chemicals |
Continue to Part II: Identification, classification and labelling of chemicals