







IPCS |
International Programme on Chemical Safety |
 Safety and Health in the Use of Chemicals at Work
Safety and Health in the Use of Chemicals at Work
A training manual
Abu Bakar Che Man and David Gold
An ILO contribution to
the International Programme on Chemical Safety
(a collaborative programme of the United Nations Environment Programme, the International
Labour Organization and the World Health Organization)
The International Labour Conference adopted, in June 1990, the Chemicals Convention (No. 170), and Recommendation (No. 177), with a view to reducing the incidence of chemically induced illness and injuries at work.
Over the past decade there has been a vast increase in the use of chemicals, and this trend will continue as chemicals have a direct impact on the improved quality of life. There are, however, many risks associated with the unsafe use of chemicals at work. Therefore safety and health in the use of chemicals offers a challenge to governments, and to employers and workers and their representatives. By strengthening tripartism and encouraging comprehensive efforts by all parties concerned, we can reduce the potential for both illness and injury and learn to work safely with chemicals.
This training manual addresses all aspects of dealing with chemicals including production, storage, use, transport and disposal. It provides an approach to problems and solutions concerning safety and health in the use of chemicals at work. Workers and their representatives will find information that will help them understand how chemicals can affect them and what measures can be taken to afford protection. Information is also provided on preventing fires and explosions. Managers and supervisors will find guidance on preventive activities and the management of a safety programme for the use of chemicals. Government officials and trainers will discover that the manual is in a format that can easily be used as the basis for a one-week training course for managers, supervisors and workers' representatives.
The manual is divided into the following chapters: the health effects of chemicals; fire and explosion hazards; methods of prevention; emergency procedures; and management of safety in the use of chemicals. These five units can be taught as a five-day course or can be presented individually, depending on the needs of the intended audience.
The ILO appreciates the direct assistance given by the Factories and Machinery Department of the Ministry of Labour of Malaysia, which provided a venue and an audience to pilot the material for this manual. Thanks are also due to Ms, Noha Karanuh, the graphic artist who illustrated the manual.
K. Kogi,
Director,
Working Conditions and
Environment Department
Fifty years ago only 1 million tons of chemical were produced annually. Little was known, and little was done, about the hazards associated with chemicals and chemical processes. Today over 400 million tons of chemicals are produced annually, and of the 5-7 million known chemical substances over 80,000 are marketed. Over 1,000 new chemicals are produced each year. It is estimated that 5,000-10,000 commercial chemicals are hazardous, of which 150-200 are considered likely to cause cancer.
Chemicals have improved the quality of life. Agrochemicals in the form of pesticides and . fertilizers have greatly increased food production. Chemotherapeutic drugs have contributed to the treatment of cancer and new drugs are constantly entering the market for the treatment of heart disease. Carbon fibres are widely used in the manufacture of new lightweight materials, while ceramic fibres are used as insulation materials and have frequently been used as a replacement for asbestos. Acrylic adhesives, superglue and environmentally safe biodegradable plastics are other examples of the important contribution of chemicals to daily life.
Today, in virtually every workplace, workers are exposed to chemicals. Chemicals such as solvents are used for cleaning and degreasing, mixing paints and varnishes, and diluting chemical fire and explosion hazards; concentrated compounds and mixtures. Chemical s in the solid state may be transformed into powders basic principles of prevention; or dust particles during the manufacturing process and may rest airborne for long periods of time. Gases and vapours are employed in industrial operations such as welding and refrigeration, or in a variety of chemical processes. Gases are also used as anaesthetic agents in hospitals. Laboratories in schools, universities, research institutions, government agencies and private enterprises make use of a variety of chemicals in both large and small quantities. In agriculture, workers may be exposed to chemical-based products such as fertilizers, pesticides and herbicides. Many chemical-based pesticides are used for controlling insect-borne diseases such as malaria. Even in today's modem office, one may find a variety of different chemicals.
However, certain chemical substances can both harm and kill. Chemicals alone in concentration, or when mixed with other chemical substances, can cause injury, disease or death. Their misuse may also result in fires and explosions. It is imperative that everyone who could potentially come into contact with chemicals should know and understand the risks, and the methods available for reducing them. This manual covers the following topics:
We hope that the information given in this manual will be of value to all those engaged in promoting or practicing the safe use of chemicals at work: managers, supervisors and workers' representatives, health and safety officers and trainers. It is written in straightforward language and assumes a minimum of technical knowledge. It is thus particularly suitable for use on training courses.
The emphasis has been placed throughout on practical guidance on the safety precautions to be taken when using chemicals at the workplace. The discussion and activities included at the end of sections and chapters may be used equally by trainers in group exercises or by individual readers in self-evaluation. Suggestions for further reading are given at the end of each chapter.
With these three concepts in mind we shall address the safe use of chemicals at work.
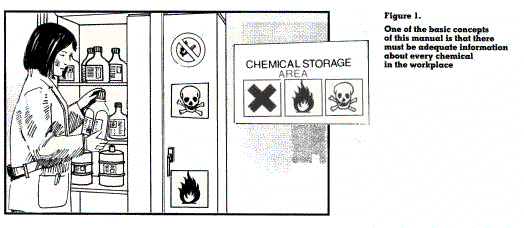
| Remember: An up-to-date label and a current chemical safety data sheet are the best sources of information on chemicals at the workplace. |
2. Health hazards due to chemical exposure
A great deal of attention in recent years has been focused on the effects of exposure to chemicals on the health of workers. Many chemicals which were once regarded as safe have been found to be associated with diseases ranging from mild skin rashes to chronic health impairment and fatal cancers. Although much has been learned about chemical toxicity from the study of diseases in laboratories and elsewhere, there are far too many chemicals used in the workplace today whose harmful effects are still unknown. It is therefore essential to treat all chemicals with care.
This chapter will explain how chemicals affect workers' health. It will enable users to recognize potentially hazardous situation, respond accordingly, and protect themselves and others. It will also allow the necessary steps to be taken to remedy the situation.
To enable users to understand the health hazards of chemicals, certain terms which are are used frequently in chemical safety are defined here:
Chemical: chemical elements and compounds, in and mixtures of them, whether natural or synthetic.
Poisoning: normally the human body is able to cope with a variety of substances, within certain limits. Poisoning occurs when these limits are exceeded and the body is unable to the deal with a substance (by digestion, absorption or excretion).
Toxicity: the inherent potential of a chemical substance to cause poisoning. The toxicity of chemicals varies widely. For example, a few drops of a given chemical will cause death while. other chemicals will produce the same effect only after a large quantity has been consumed.
Hazard: a potential to cause danger to life, health property or the environment.
Chemical hazard: any chemical that has been classified as hazardous or for which relevant information exists to indicate that it is hazardous.
Risk: the measured probability of an event to cause danger to life, health, property or the environment.
Airborne dust: refers to the suspension of solid particles in the air. These dust particles are generated by handling, grinding, drilling and crushing operations where solid materials are broken down. The size of these particles ranges from being visible to the naked eye (i.e. greater than one twentieth of a millimeter in diameter) to being invisible. Invisible dust will remain airborne for a long period of a time and is dangerous because of its ability to penetrate deeply into the lungs.
Vapour: the gaseous form of a liquid at room temperature and pressure. Liquids emit vapours, the quantity depending on their volatility. Substances with a low boiling-point are more volatile than those with a higher one.
Mist: the dispersion of liquid particles in the air. Mists are normally generated by processes such as electroplating and spraying where liquids are sprayed, splashed or foamed into fine particles.
Fumes: solid particles formed from condensation of substances from vapour state. Fumes are normally associated with molten metals where the vapours from the metal are condensed into solid particles in the space above the molten metal. The size of the particles are in the range visible to the naked eye.
Gas: a substance, such as oxygen, nitrogen or carbon dioxide, which is in the gaseous state at room temperature and pressure.
Acute effect: the effect caused by a single short term exposure (usually not more than one work shift) to a high amount or concentration of a substance.
Chronic effect: the effect caused by repeated exposure to a chemical over a long period of time. The effect may be felt only after many years of exposure. Both acute and chronic effects can be reversible after the termination of the exposure and appropriate treatment, or they may result in long lasting, irreversible conditions.
Chemical safety data sheet: a document containing essential information ma ion for users regarding the properties of chemicals classified as hazardous and methods of using them safely, including their identity, supplier, classification, hazards, safety precautions and emergency procedures.
2.2. Factors contributing to hazardous situations
Many factors can influence the intensity of hazards associated with chemicals in the workplace. These include toxicity, the physical properties of the substances, work practices, the nature of the exposure, combined exposures, the routes of entry, and the susceptibility of workers. It is important to understand how these factors, in combination, contribute to hazardous situations.
Chemicals can enter the body in three ways. In the workplace, the inhalation of gases, vapours or airborne particles and absorption through the lungs is the most important route of entry. However, a number of chemicals, particularly liquids, can be absorbed through intact skin when coming into direct contact with it. The ingestion of poisons through the mouth is common where personal hygiene is poor.
In industry, inhalation is the most significant route of entry The respiratory system represents an efficient entry point for chemicals. With a total surface area of the lungs of 90 square meters in a healthy adult, a worker performing a moderate task inhales about 8.5 cubic meters of air in the course of an eight-hour shift.
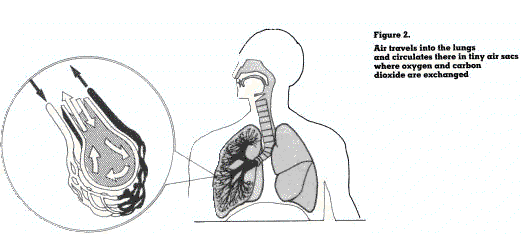
The respiratory system consists essentially of the upper respiratory tract (nose, mouth, throat), the air passageways (trachea, bronchi, bronchioles, alveolar ducts) and the gas exchange area (alveoli) where oxygen from the air diffuses into the blood and carbon dioxide from the blood diffuses into the air (figure 2).
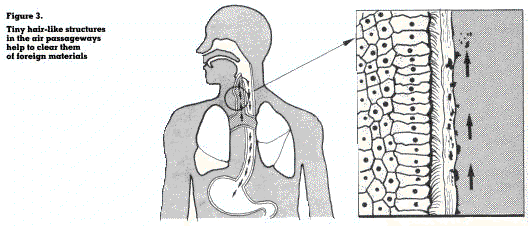
The air passageways are lined with tiny hairlike structures (cilia). These structures are part of the clearing mechanism of the lungs which causes foreign particles, deposited on the surfaces of the respiratory passages within the lungs to be carried by mucus towards the throat (figure 3). It is estimated that 2 liters of mucus flow to the throat each day.
During breathing, airborne chemicals enter the nostrils or mouth, pass through the air passageways and finally reach the gas exchange area where they are either deposited or pass through the wall of the area into the bloodstream.
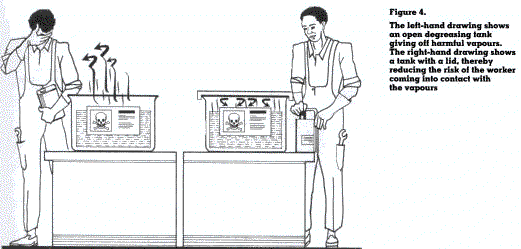
Certain substances irritate the mucous membrane of the upper respiratory tract and respiratory passages within the lungs. This irritation may serve as a warning of the presence of chemicals. However, certain gases or vapours do not have this effect. Unnoticed by the workers, they penetrate deeply into the lungs causing lung injury, or become transported in the bloodstream (figure 4).
The entry of dust particles into the body depends on their size and solubility. Only small particles (less than seven thousandths of a M111imetre in diameter) will be able to reach the gas exchange area. This respirable dust (which reaches the gas exchange area) will either be deposited there or diffused into the bloodstream, depending on the solubility of the chemicals. Insoluble dust particles are mostly eliminated by the clearing mechanisms of the lungs. The larger dust particles are filtered by the hairs of the nostrils or deposited along the path from the nose to the air passageways. They will eventually be transported to the throat where they will be either swallowed, or spat or coughed out.
| Remember: Extreme care must be taken because chemicals in the form of vapour, fumes, dust or gas can easily enter the body through breathing. |
Discussion questions:
Ingestion is another way in which chemical substances can enter the body. Entry via ingestion is possible when workers eat or smoke with contaminated hands or eat their meals at their workstation where food and drink may be contaminated by vapours in the air (figure 5).

A second way in which chemical substances are ingested is when inhaled particles are transported to the throat by the air passageways into the lungs, and swallowed.
The digestive system consists of the oesophagus, the stomach, and.the small and large intestine. Absorption of food and other substances, including ingested hazardous chemicals, occurs primarily in the small intestine.
| Remember: If you eat or drink at your workstation, you may be introducing hazardous chemicals into your digestive system because the substance may coat the food or eating utensils. |
Discussion questions:
Absorption through the skin constitutes another route of entry. The thickness of the skin, together with its natural covering of sweat and grease, provide some protection against chemical exposure.
The solubility of chemicals (such as organic solvents and phenol) in fats enables their absorption through the skin. If the skin is damaged by cuts or abrasions, or is diseased, the chemical would be absorbed into the body even quicker.
Discussion questions:
2.2.2. Concentration and type of exposure
Chemical substances that enter the body through inhalation, ingestion or skin absorption will leave the body through the urine. Others will be excreted unchanged via breathing or urination. These substances may cause damage to internal organs. The breaking down and detoxification of certain substances (normally occurring in the liver) may produce by-products or new substances which may be more harmful than the original ones. The damage done by a chemical to a specific organ depends in principle on the amount (dose) absorbed. In the case of inhalation, the dose depends mainly on the concentration of the substance in the air and the duration of the exposure. Therefore a short-term exposure to a high-level concentration may result in acute effects (acute poisoning ' oning), whereas exposure to a low concentration spread over a long period of time, which would result in the same absorbed amount of the toxic substance, may be tolerated but may result in an even higher cumulative dose resulting in chronic effects.
2.2.3. Combined effects of chemicals
Occupational exposure is rarely confined to a single chemical. In most instances workers are are transported by the bloodstream. Some of these exposed to two or more chemicals. The combined chemicals will be stored in tissues or organs, with effect of multiple exposure to chemicals is an area very little being excreted. Some will be changed for which sufficient information is often lacking. It into, other substances which are more soluble and may happen that a combination of two chemicals, by chemical reaction or absorption into the body, produces a new substance with totally different properties and even more harmful to health than the chemicals acting separately (figure 6).
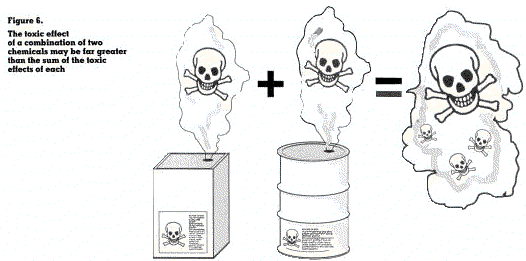
Because of this lack of information on the combined effects of chemicals, multiple exposure should be avoided or reduced to the lowest possible level.
| Remember: Avoid mixing several chemicals together. The combination may result in very dangerous effects. |
2.2.4. Hypersusceptible groups
There is great variation in individual response a chemical. Exposure to a particular dose over a similar time period will induce different responses among different people. Some may be severely affected and some may be mildly affected, while others may show no apparent effects. Individual sensitivity may also depend on age, sex and general state of health. Children, for example, will be more sensitive than adults. The unborn foots may be very susceptible to the risks of chemical substances. Therefore, in the recognition of potential hazards, individual variation in sensitivity should be taken into account. skin, the eyes and the respiratory tract.
Discussion questions:
2.3. The toxic effects of c:chemicals
As explained above, the effects of chemicals can be either acute or chronic, depending on the concentration and length of exposure. Chemicals may also produce different effects for different modes and types of exposure. The effects of chemicals can be categorized into the following groups:
Irritation means a condition that is aggravate when chemicals come into contact with the body'. The parts of the body normally affected are the skin, the eyes and the respiratory tract.
When certain chemicals come into contact with the skin, they can remove the protective layer causing it to become dry, rough and sore. This condition is called dermatitis (figure 7). There are many chemicals causing dermatitis.
Figure 7. Many chemicals
cause dermatitis by coming into contact with the skin
Contact of chemicals with the eyes may cause skin damage ranging from mild temporary discomfort to permanent damage (figure 8).
The severity of damage depends on the dose and the speed with which first-aid measures are administered. Examples of substances causing eye irritation are acids, alkalis and solvents.
Irritants in the form of mist, gas or vapour will induce a burning sensation when in contact with the upper respiratory tract (nose and throat). This is normally caused by soluble substances such as ammonia, formaldehyde, sulphur oxide, acids and alkalis which are absorbed by the moist lining of the nose and throat. Care should be taken that these vapours are not inhaled when workers are dealing with chemicals, for example during spraying (figure 9).
Some irritants exert their effects along the conducting airways, causing bronchitis and sometimes serious damage to the lining and the lung tissues. Examples are sulphur dioxide, chlorine and coal dust.
Chemicals which are less soluble in water will penetrate to the gas exchange areas, causing serious irritation effects. Their presence in the workplace is not normally detected and can present serious hazards to workers. The reaction of chemicals with the lung tissues induces pulmonary oedema (fluid in the lungs), either immediately or after a few hours. The symptoms begin with intense irritation followed by coughing, dyspnoea (shortness of breath), cyanosis (lack of oxygen) and expectoration of large quantities of mucus. Examples are nitrogen dioxide, ozone and phosgene.
Discussion questions:
An allergy can be acquired through exposure to chemicals. Initially workers may not develop an allergy; constant exposure, however, may produce a reaction by the body. Even exposure to a low level of a substance could later induce an allergic reaction. Either the skin or the respiratory tract can be affected by an allergic reaction.
An allergic reaction of the skin is a condition that often looks like dermatis (small pimples or watery blisters). This effect may not appear at the site of contact but can occur elsewhere on the body. Examples of sensitizers are epoxy resin, amine hardeners, azo dyes, coal tar derivatives and chromic acids.
Sensitization of the respiratory tract causes, occupational asthma. The symptoms of this reaction often include coughing, especially at night, and difficulties in breathing such as wheezing and shortness of breath. Examples of chemicals causing this type of reaction are toluene diisocynate and formaldehyde.
| Remember: Repeated exposures to chemicals may lead to allergic reactions. |
2.3.3. Lack of oxygen (asphyxiation)
Asphyxiation refers to interference with the oxygenation of the body tissues. There are two types: simple and chemical asphyxiation.
This refers to a condition whereby oxygen in the air is replaced by an inert gas such as nitrogen, carbon dioxide, ethane, hydrogen or helium to a level where it cannot sustain life. No, mal air contains about 21 per cent of oxygen. If this concentration falls below about 17 per cent, the body tissues will be deprived of their supply of oxygen, causing symptoms such as dizziness, nausea and loss of coordination. This type of situation may occur in confined workplaces (figure 10). A further reduction of oxygen may cause unconsciousness and death.
2.3.3.2. Chemical asphyxiation
In this situation direct chemical action interferes with the body's ability to transport and use oxygen. An example of a chemical asphyxiant-- is carbon monoxide. Concentrations of 0.05 per cent of carbon monoxide in the air may considerably reduce the capacity of the blood to carry oxygen to the various tissues of the body. Another example is the toxic effect of hydrogen cyanide or hydrogen sulphide. These substances interfere with the cells' ability to accept oxygen even though the blood is rich in oxygen.
Discussion questions:
2.3.4. Narcosis and anesthesia
Exposure to relatively high concentrations of certain chemicals such as ethyl and propyl alcohols (aliphatic alcohol), acetone and methylethyl ketones (aliphatic ketone), acetylene hydrocarbons, and ethyl and isopropyl ethers depresses the central nervous system. These chemicals will induce an effect similar to being drunk. Single exposure to a high concentration may result in unconsciousness or even death. There are also cases where workers have become addicted to these substances.
The human body is made up of many systems. Systemic poisoning refers to the adverse response induced by chemicals to one or more body systems, which in turn spreads throughout the body. The effect is not localized at any one spot or area of the body.
One of the tasks of the liver is to purify any noxious substances from the blood by converting them to harmless and water-soluble substances before being excreted (figure 11). However, some chemical substances cause damage to the liver. Depending on the dose and frequency of exposure, repeated damage to liver tissues may cause injury resulting in scarring (cirrhosis) and decreased liver function. Liver injury can be caused by chemicals such as solvents (alcohol, carbon tetrachloride, trichloroethylene, chloroform) and may be mistaken for hepatitis, as the symptoms (yellowish skin and eyes) produced by these chemicals are similar.
The kidneys are part of the urinary system. Their task is to excrete waste products generated by the body, maintain the balance of water and salts, and control and maintain the acidity level of the blood (figure 12). Chemicals that prevent the kidneys from excreting poisonous products include carbon tetrachloride, ethylene glycol and carbon disulphide. Other chemicals such as cadmium, lead, turpentine, methanol, toluene and xylene will slowly deteriorate the kidney function.
The nervous system controls body function (figure 13), and can be damaged by certain chemicals. Chronic exposure to solvents has been linked to symptoms such as fatigue, sleep difficulties, headache and nausea. More serious cases cause motor disturbances, paralysis and an impaired sense of perception. Exposure to hexane, manganese and lead has been associated with effects on the peripheral nerves resulting in symptoms of "wrist drop". Exposure to organophosphate compounds such as parathion may cause the nervous system to fail. Another example is carbon disulphide, which has been linked with cases of mental disorder (psychosis).
Figure 13. The nervous system.
composed of the brain,
the spinal card and the nerves, controls body functions and can be affected by chemicals
Exposure to certain chemicals may also have negative ef f ects on the reproductive system, producing sterility in men and causing miscarriages in pregnant women. Chemicals such as ethylene dibromide, benzene, anaesthetic gases, chloroprene, lead, organic solvents and carbon disulphide have been linked with the reduction of fertility in male workers. Miscarriages are linked with exposure to anaesthetic gas, mercury ethylene oxide, glutaraldehyde, chloroprene, lead, organic solvents, carbon disulphide and vinyl chloride.
Long exposure to certain chemicals may cause an uncontrolled growth of cells, resulting in cancerous tumours. These tumours may appear many years after the first exposure to the substances. This period is called the latency period and may range from four to 40 years. The site of occupational cancer varies and may not necessarily be confined to the contact area. Substances such as arsenic, asbestos, chromium, nickel and bischloromethyl ether (BCME) may cause lung cancer. Cancer of the nasal cavities and sinuses is caused by chromium, isopropyl oils, nickel, wood and leather dust. Cancer of the bladder is linked with exposure to benzidine, 2-naphthylamine and leather dust. Skin cancer is linked to exposure to arsenic, coal tar and petroleum products. Liver cancer can be caused by exposure by vinyl chloride monomer, while cancer of the bone marrow is caused by benzene.
2.3.7. Damage to the unborn foetus (teratogenesis)
Congenital malformation resulting from exposure to chemicals may interfere with the development of the normal foetus. During the first three months of pregnancy, the vital organs such as brain, heart, arms and legs are forming. Studies conducted have suggested that in the presence of certain chemicals such as anaesthetic gases, mercury and organic solvent, the normal process of cell division may be interfered with, causing deformity in the foetus.
2.3.8. Genetic effects on future generations (mutagenesis)
The genetic effects of certain chemicals on workers may lead to undesired changes in future generations. Information on these effects is scarce. However, results of laboratory tests suggest that 80 to 85 per cent of carcinogenic chemicals may also have effects on future generations.
2.3.9. Dusty lung (pneumoconiosis)
Dusty lung, or pneumoconiosis, is a condition caused by the deposit of small dust particles in the gas exchange areas of the lung and the reaction of the tissue to their presence. Changes in the lungs are extremely difficult to detect at the early stage, and deterioration occurs long before such changes can be detected by X-rays. With pneumoconiosis the capability of the lungs to absorb oxygen will be reduced and the victim will develop shortness of breath during strenuous activities. The effect is irreversible. Examples of substances causing pneumoconiosis are crystalline silica, asbestos, talc, coal and beryllium.
Discussion questions:
Suggested further reading:
Chemicals in the workplace may pose certain risks of fire and explosion. The improper storage, transport, use or disposal of these chemicals may result in an event ranging from a minor fire to a disaster causing major economic loss, suffering and loss of human life.
This chapter will address some of the risks of fire and explosion due to chemicals in the workplace. Chapter 4 discusses some preventive actions. Above all, it is important to remember that the prerequisite to understanding the safe use of chemicals in the workplace is an adequate knowledge of them through chemical safety data sheets and easy identification through proper labeling.
This chapter will also help you understand some of the basic fire and explosion information that is found on the chemical safety data sheet.
In order for human beings to survive, at least three basic elements are essential: food, oxygen and heat. These elements must also be in the right proportions for human survival. Too much or too little food, oxygen or heat may result in discomfort, illness or death.
In the same way, fire needs three elements: fuel, oxygen and a source of heat. These must be in the right proportions and the right states before fire can be ignited and before it can continue to burn. The fuel must be at a temperature, defined as the flashpoint, at which flammable vapours are released. Therefore there must be enough heat to bring the fuel to this point. There is also a need for oxygen. Normally, fire needs 15-21 per cent oxygen to ignite and burn.
Discussion questions:
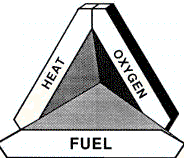
When examining the risks of fire and explosion resulting from hazardous chemicals, one must first look at the chemical and its characteristics. Most often the chemical substance will act as the source of fuel in the fire or explosion triangle (figure 14).
Figure 14. Fuel is the first element of the fire and explosion triangle
One characteristic of chemicals that pose a fire or explosion risk is the flashpoint. This is the lowest temperature at which a chemical gives off flammable vapours. It is the flammable vapour that burns, not the liquid. Table 1 shows the flashpoints of some commonly used chemicals.
Table 1. Some common flashpoints
| Chemical | Flashpoint (oC) |
| Gasoline | -43 |
| Acetone | -19 |
| Methyl alcohol | 11 |
| Kerosene | 43 |
| Heptane | -4 |
| Toluene | 6 |
| Remember: In terms of fire and explosion, the lower the flashpoint the more dangerous the chemical. |
Other factors may relate to a chemical's ability to reach its flashpoint. For example, when a liquid such as kerosene is atomized, it will produce flammable vapours which will bum at a lower ambient temperature than its flashpoint. In addition, a chemical with a high flashpoint may be heated to its flashpoint by other substances with lower flashpoints burning in close proximity to the first substance. It is therefore essential that careful consideration should be given to the safe storage of hazardous chemicals.
When a chemical is heated to its flashpoint, the flammable vapours will bum only until the heat source is removed. However, once the liquid reaches its firepoint (normally only a few degrees above the flashpoint), the vapours will continue to be produced and bum. The flashpoint is normally found on the chemical safety data sheet.
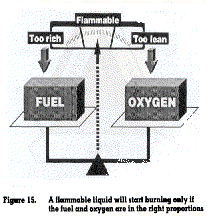
3.1.1.2. Upper and lower explosive limits
Flammable liquids must also have the right mixture of oxygen to bum (figure 15). An excess of fuel with an inadequate amount of oxygen may mean that the substance is too rich to burn. Conversely, a high concentration of oxygen with an inadequate amount of fuel may mean that the substance is too lean to bum. The limits at which a substance will ignite, depending on the percentage of oxygen, are called the upper and lower explosion limits (UEL and LEL). The UEL and LEL are normally found on the chemical safety data sheet.
An additional consideration is the weight of the chemical as compared with the relative weight of air. This is known as the vapour weight. Gasoline vapours, for example, are three-and-half times heavier than air. Other chemical substances whose vapour weights are heavier than air include kerosene, carbon disulphide, acetylene and carbon monoxide. When working with a chemical that is heavier than air, it is important to know that its vapours may travel long distances and form concentrations at a considerable distance from their source, often at a low point such as a cellar.
| Remember: Vapours of chemicals that are heavier than air may travel long distances and concentrate in cellars. |
Certain chemicals, in a solid state, will bum rapidly once ignited. Magnesium, for example, will bum once ignited and will be very difficult to extinguish. Fuels in the form of dusts or powders may also explode in the right mixture of oxygen. When agitated and an appropriate ignition source is present, these dusts and powders will bum explosively creating multiple sequential explosions as additional dusts or powders are agitated.
Gases, of which several are very flammable, are commonly used in industry. Acetylene, hydrogen and methane (often a by-product) will explode in the right concentration of gas and oxygen when an ignition source is present.
Caution must also be exercised in dealing with compressed gases stored in pressurized containers. These gases, when heated within the containers, may expand to a point where the container fails, frequently resulting in a disastrous situation.
Discussion questions:
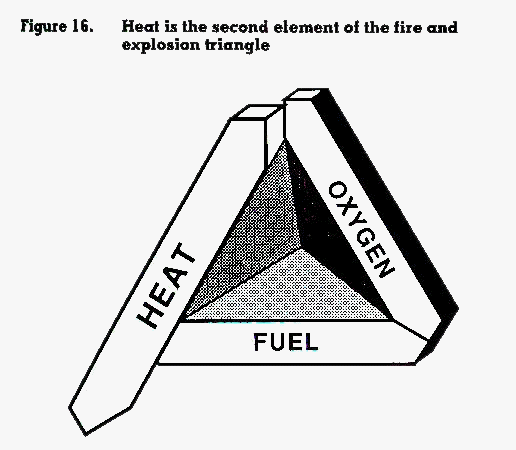
Heat is the second element of the fire or explosion triangle (figure 16). It is needed to bring the fuel to its flashpoint (if the flashpoint is above the ambient temperature) and to ignite the flammable vapours. Sources of heat that can ignite hazardous chemicals include electrical current, static electricity, spontaneous combustion, chemical reaction, friction, process heat, open flames, solar heat, radiant heat (hot surfaces) and lightning. One of the keys to the prevention of fires and explosions caused by hazardous chemicals is the control of sources of heat. The control aspects will be discussed in Chapter 4.
Heat is generated through electrical current in three ways: resistance, arcing and sparking. Resistance occurs when electricity travels through wires not large enough to carry the current. The result is either a blown fuse or a tripped circuit breaker, or the heating of the wire in the circuit.
This circuit may reach a high enough temperature to ignite flammable vapours present in the air or may cause combustible materials to ignite, bum and elevate the temperature of nearby chemicals to their flashpoint and their firepoint. Electrical arcing happens when electrical current jumps from one point to another. This may occur in a switch or connection box when wires separate from connectors or when the insulation of wires is worn away between a positive and a neutral wire (e.g. when temporary wiring or extension cords have been exposed to forklift trucks or when workers constantly tread on the wire and wear away the insulation). The resulting electrical arc can ignite flammable vapours. Molten metal resulting from the arcing can also ignite combustible materials, thus causing the heating of chemicals as above. Sparking may also ignite flammable vapours that are present.
Static electricity is generated when two dissimilar surfaces come together and are separated, resulting in the build-up of positive and negative charges (figure 17).
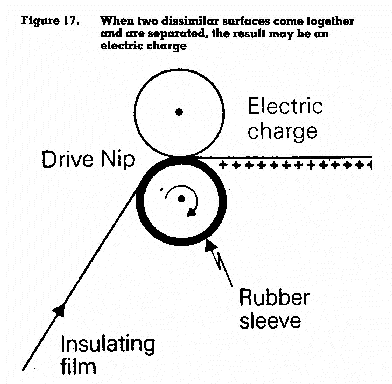
The resulting spark may cause the ignition of flammable vapours or an explosion. For example, in machines which process film and sheet material, insulating material becomes charged by passage through a machine (figure 17). If such materials continue to be processed in flammable atmospheres, the charges created should be carefully neutralized to avoid sparking. Static build-up can occur when two surfaces rub together, or when liquids are transferred from one container to another without proper earthing and bonding (a common source of explosions when flammable liquids are transferred).
This phenomenon has been known to occur in industry when piles of oily rags have been left to dry in the open air. Certain kinds of oils tend to produce heat, as they are oxidized, and may create a fire in the pile of rags. (A similar situation may occur in agriculture by the heat created by fermentation when wet hay is baled and stored.) The simple measure of storing oily rags in covered containers (thus reducing the amount of oxygen) diminishes this risk.
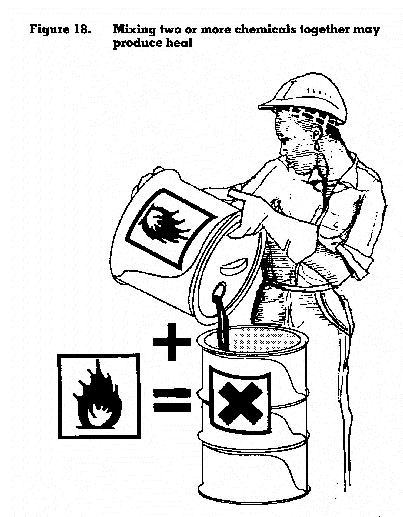
3.2.4. A mixture of two chemicals
As stated in Chapter 2, when two or more chemicals are mixed, the combined effect can be more dangerous than the sum of their separate effects. This combined effect may also carry a greater risk of fire and explosion. For example, the mixed chemicals may have a lower flashpoint and a lower boiling point, and may give off eas4y ignitible vapours.
The reactions of two chemicals coming together could possibly produce sufficient heat, as a by-product, for other chemicals in the vicinity to be heated to a point where they become dangerous (figure 18). Thus a chain reaction could be started that could have catastrophic results.
When two surfaces rub together, heat may be produced. This is known as friction. Drive belts nibbing against their housings or guards, or metal surfaces rubbing against each other, may generate an adequate amount of heat to ignite flammable vapours. Friction is frequently due to a lack of adequate maintenance, resulting in loose guards or inadequately lubricated metal surfaces or joints. A spark can also occur when a stone lodged in the sole of a shoe strikes a concrete surface.
Heat from furnaces, vats, cooking stoves and other hot surfaces may ignite flammable vapours. The normal manufacturing process may also cause the production of sufficient heat to bring the chemicals stored in the vicinity to their flashpoint and to ignite the vapours. The direct rays of the sun, either by themselves or magnified by plastic or glass, can also have this effect.
| Remember: Production of heat may cause chemicals to reach a temperature where flammable vapours are present. The heat may also ignite the flammable vapours, causing a fire or explosion. |
Unprotected flames caused by cigarettes, matches, welding torches and internal combustion engines are very important sources of heat. When coupied with adequate fuel and in the presence of oxygen, they can create a fire or explosion (figure 19)

Discussion question:
List at least three sources of heat that may ignite flammable liquids in your workplace.
Oxygen is the third element of the fire or explosion triangle (figure 20). Most fuels need at least 15 per cent oxygen to burn. In excess of 21 per cent, oxygen may cause more rapid intense combustion leading to explosions. Sources of oxygen other Than the environment may include oxygen cylinders for cutting and welding operations, oxygen supplied by piped-in manifolds for process operations, and at times chemical reactions. Oxygen released by a chemical when heated is known as an oxydizer; some examples are given in table 2.
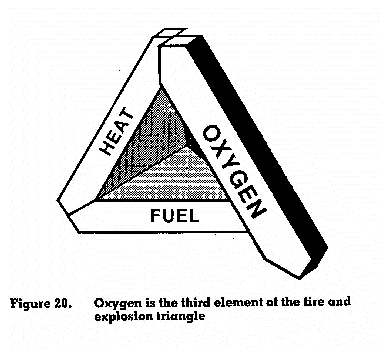
Table 2. Examples of chemicals that give off oxygen when heated
| Nitrates | Ammonium nitrate and sodium nitrate |
| Nitrites | Ammonium nitrite |
| Inorganic peroxide | Hydrogen peroxide |
| Permanganates | Potassium permanganates |
Discussion questions:
Suggested further reading
National Fire Protection Association: Fire prevention handbook (Quincy, Massachusetts, 17th ed., 1991)