







IPCS |
International Programme on Chemical Safety |
CHEMICAL SAFETY TRAINING MODULES
PART IV: TRANSPORT AND STORAGE
1. What happens during the transport of chemicals
Transport is necessary for products to reach consumers and the raw materials to the production site. The transport and storage of dangerous chemicals and goods has increased with technical development and production development.
An accident occurring during the transport of dangerous goods can lead to catastrophic consequences: laws and recommendations have been established to protect the society and the environment. But they can not be effective if you, whether you are an employer, worker, transporter or inspecting authority, do not share the responsibility and follow existing recommendations and guidelines of transport and storage, in order to avoid unnecessary risks.
The hazardous properties of products or chemicals should be clearly stated so that people of all stages of the transport chain are aware of them. This information should always follow the goods so that people can recognize the risks, avoid accidental mishandling and have the right kind of the personal protection at their disposal in case of leakage.
Dangerous goods can be transported without causing unnecessary hazards if handled properly and with care.
Dangerous goods can be explosive, flammable, toxic, radioactive, corrosive or harmful in some other way to humans, animals or the environment. Here the environment includes also other goods in transport, the vehicle, buildings, soil, roads, air, waterways and nature in general.
The empty containers and packages of dangerous goods can present the same hazards as the chemical substance or product they contained and should also be regarded as dangerous goods.
United Nations statistics show that half of transported goods belong to the category of dangerous goods. Sea transports of petroleum products by tankers form a large proportion of all transported goods. The amount of road and railway transport is also significant. For example, 85% of chlorine, which is one of the very dangerous chemicals, is transported by rail.
Large amounts of other highly dangerous goods, such as hydrochloric acid, sulphuric acid, sulphuric dioxide, nitric acid, phenol and methanol are transported regularly.
Major accidents cause extensive damage. We forget easily that small amounts of oil, gasoline, battery acids and refrigerator fluids are released to environment daily. Even small but frequent wastes from ships, households, cars or agriculture increase the load to the environment. For example one litre of oil can, under unlucky circumstances, spoil 100 000 litres of drinking water. A spill of hydraulic fluid from a truck can lead to environmental damages on the site.
To avoid harmful or dangerous circumstances recommendations and instructions for the handling, storage and transport of dangerous goods must be clear and unambiguous. Transport of dangerous goods does not pose under normal conditions a greater danger than any other transported goods if the responsible persons in the transport chain respect the existing recommendations and laws and respect the type of the hazards of the cargo.
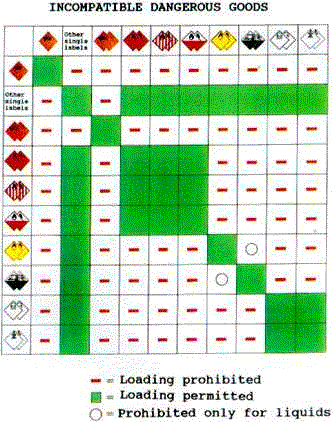
| There is always a risk of spillage during the transport of
hazardous goods. When incompatible substances mix with each other there is a possibility
of a chemical reaction, which can produce enough heat to cause fire or explosion and can
release dangerous gases. Spillage is possible in the following situations:
A risk of an accident is present when
A chemical substance or preparation may be hazardous when it comes into contact with other chemicals including air, water or humidity. For example, when calcium carbide (used in the production of acetylene and pyrotechnics) comes to contact with water, it releases the extremely flammable gas of acetylene (also in welding flame) and creates an explosion hazard. Careful handling is also important because the magnitude of the involved risk is not always obvious. One kilo of a certain chemical poses a hazard, but it is not obvious that ten kilos creates a ten fold hazard. The danger could be the same or higher as for one kilo. |

|
The pressure within sealed packages rises in the heat, caused, for example, by sunshine, and can lead to uncontrollable reactions. Changes in temperatures may affect both the qualities of a cargo and its packing material. The recommendations on package sizes as well as the load size should be respected.
Common hazards in handling of chemicals are
Special conditions can increase the risks
Many companies allow uncontrolled access of diesel engines into premises believing that they cannot ignite gas or vapour. This is incorrect:
Four tons of hot, flammable hydrocarbon leaked out of the a plant while maintenance work was in process. A diesel engine was running in the area. The flammable vapour was sucked into the air inlet and the engine started to race. The driver tried to stop the engine by stopping the fuel supply (usual way of stopping a diesel engine) but without success as burning material was coming in through the air inlet. Finally there was a flash-back and the flammable liquid was ignited to a fire.
Another frequent incident is this type:
a tank trailer tipped up because of the rear compartments were emptied first. If it is not possible to keep trailer connected to the truck's driving unit the front compartments should be filled last and emptied first as the normal support cannot alone prevent the trailer from tipping.
| The United Nations has published a book collecting the work
of the Committee of Experts: Recommendations on the Transport of Dangerous Goods. These
recommendations aim to present a basic, practical scheme of provisions that will allow
national and international regulations governing various modes of transport to develop
within it in a certain uniformity. The aim is to enable effective and successive transport
and to ensure the safety of people, property, and the environment. In these recommendations the goods are given an identification number and are divided to the following classes describing the inherent hazards: 1. EXPLOSIVES 1.1 Substances and articles which have a mass explosion hazard 1.2 Substances and articles which have a projection hazard but not a mass explosion hazard 1.3 Substances and articles which have a fire hazard and either a minor blast hazard or a minor projection hazard but not a mass explosion hazard 1.4 Substances and articles which present no significant hazard 1.5 Very insensitive substances which have a mass explosion hazard 1.6 Extremely insensitive articles which do not have a mass explosion hazard 2. GASES 2.1 Flammable gases 2.2 Non-flammable, non-toxic gases 2.3 Toxic gases 3. FLAMMABLE LIQUIDS 4. FLAMMABLE SOLIDS 4.1 Flammable solids 4.2 Substances liable to spontaneous combustion 4.3 Substances which in contact with water emit flammable gases 5. OXIDIZING SUBSTANCES; ORGANIC PEROXIDES 5.1 Oxidizing substances 5.2 Organic peroxides 6. POISONOUS (=TOXIC) SUBSTANCES 6.1 Toxic substances 6.2 Infectious substances 7. RADIOACTIVE MATERIAL 8. CORROSIVE SUBSTANCES 9. MISCELLANEOUS DANGEROUS SUBSTANCES The properties of the chemical substance or product have an effect on the choice of the packing material. Recommendations on the material, as well as the sizes, of packages are based on testing and experience. Dangerous goods of classes 3, 4, 5.1, 6.1, 8, and 9 have been divided for packing purposes into three groups according to the degree of danger they present:
|
|
Packing Group I: Substances and preparations presenting a very severe toxicity risk
Packing Group II: Substances and preparations presenting a serious toxicity risk
Packing Group III: Substances and preparations presenting a relative low toxicity risk
The packing group related to a special substance, together with advice on packing methods, is listed in `UN Recommendations on the Transport of Dangerous Goods' and in many national regulations and provisions.
To deal with goods having multiple risks a subsidiary risk classification is used together with the principal hazard classification. Substances and articles may often have more than one hazardous property and are subject to further restrictions.
These goods may be
Examples of hazard classes:
Substance or article |
Hazards |
||
| UN Number | Name and description | Class | Subsidiary risk |
| 3017 | Organophosphorus pesticides, liquid, toxic,
flammable, flash-point not less than 23oC (Demeton, Fenthion, Parathion) |
6.1 | 3 |
| 1396 | Aluminium powder, uncoated | 4.3 | |
| 1005 | Ammonia, anhydrous | 2.3 | 8 |
| 1789 | Hydrochloric acid | 8 | |
| 1011 | Butane | 2.1 | |
This class contains articles, preparations, and substances such as ammunition, TNT, dynamite, nitrourea, fireworks.
A transport accident involves acute risk of explosion. The pressure wave can be devastating, and flying splinters may cause great damage.
The heat of the blast can result in a fire.
Some substances in this class have toxic properties, e.g. nitroglycerin (in dynamite) is also classified as toxic and can penetrate through the skin.
Transport of Class 1 articles or substances are subject to many restrictions, including quantity and temperature limits. They may also be incompatible with other goods. For example, dynamite should not go with detonators.
This class contains
The term "compressed" refers to gases under pressure but not in a liquid state. Gases are usually stored in cylinders. When the valve is opened or broken, gas alone is released. The pressure of the cylinder depends on the type of gas it contains. The cylinders should always be kept within the approved temperature range to avoid a risk of overpressure causing an explosion hazard. Nitrogen (Class 2.2), hydrogen (2.1), oxygen (2.2 and 5.1) and helium (2.2) are compressed gases.
Condensed gases are in a liquid state at relative low pressure. The contents are released as liquids which quickly evaporate forming gas clouds. The size of the cloud can be considerable; for example, 1 litre of liquified petroleum gas (LPG) forms up to 250 litres of gas. LPG, (propane, butane or a mixture of them: cooking gas)(Class 2.1), ethylene (2.1), vinyl chloride (2.1), halogenated hydrocarbon gases (freons)(2.2), carbon dioxide (2.2), chlorine (2.3 and 8), ammonia (2.3 and 8) are commonly used gases in industry and are transported as condensed gases.
Some condensed gases are stored at very low temperatures. They are transported in well- isolated containers called dewars. These must have a loosely covered opening to avoid dangerous overpressure. They pose special hazards due to their low temperature. For example, splashes of liquified nitrogen can cause frostbite and the gas cloud is an asphyxiant.
In a train accident several tank wagons filled with liquified propane turned over spilling their contents. The propane started to evaporate cooling the surroundings to 43oC, which is the boiling temperature of propane. Several persons in the spill area were frozen to death. With good luck and tight security the highly flammable propane gas did not explode.
An example of a dissolved gas is acetylene (Class 2.1). Acetylene cylinders are filled with inert very porous, non-combustible material which is wetted with acetylene.
Aerosols and small receptacles which contain flammable propellant gases belong to this class.
Substances in Class 2 are assigned to one of three categories based on the primary hazard of the gas.
2.2.1. Class 2.1: Flammable gases
This category includes those gases that at normal pressure and temperature, as a mixture of 13% or less with air, can ignite from a source of fire.
2.2.2. Class 2.2: Non-flammable, non-toxic gases
Gases in this category might replace oxygen and are asphyxiant (nitrogen, carbon dioxide), or are oxidizing and may contribute to the combustion of other materials more than air does. Pure oxygen is an example of gas enhancing fires.
Gases which are known to be poisonous or corrosive enough to pose a health hazard belong to this category (carbon monoxide, ethylene oxide, hydrogen sulphide, sulphur dioxide and ammonia). Containers with toxic gases should never be loaded or stored together with food or feedstuffs.
Acidic gases can react with alkaline gases to produce heat and smoke, which may create a fire risk.
Some gases have more than one dangerous property. They can be both flammable and toxic (methyl ether) or corrosive and toxic (hydrogen chloride, phosgene, chlorine).
2.3. Class 3: Flammable liquids
A flammable liquid has the ability to give of, at normal temperatures, vapours which are flammable. Benzene, kerosene, toluene, propanol and various organic solvents used in pesticides are examples. This class covers mixtures of liquids, as well as liquids containing solids in solution or suspension (paints, varnishes, lacquers, etc.). Petroleum products and crude oil also belong to Class 3.
Flammable liquids pose a risk of fire and explosion, and may lead to expensive environmental clean-up operations (accidents at sea to oil tankers).
The flammability of a liquid depends on several characteristic properties.
Flash-point describes the lowest temperature at which a liquid gives off sufficient amount of flammable gas to form a mixture with air which will ignite when a flame or spark is present. If the flash-point is tested to be not more than 60.5oC, the substance belongs to Class 3.
Many flammable liquids can be charged with static electricity, for example, as result of flowing in a pipe. This makes them both combustible and able to create a spark. Containers should have an earth connection in situations such as refilling the cisterns at petrol stations.
Some flammable liquids have more than one dangerous property. Carbon disulphide is both flammable and toxic, and formaldehyde solutions can be both flammable and corrosive.
2.4. Class 4.1: Flammable solids
This class consists of solids which are readily combustible, those which may cause or contribute to fire through friction, and self-reactive substances. Sulphur and red phosphorus are common hazardous substances in this class. Examples of self-reactive compounds are azocarbamides, benzene sulphohydrazine and diazonium salts. Sawdust, hay and paper are not spontaneously flammable but are in this class because of incompatibility requirements in loading procedures.
When flammable solids are handled there is a possibility of large amounts of dust being released in the air. These mixtures of dust and air can lead to a dust explosion.
Many flammable solids give off hazardous fumes when they burn. For example, the fumes of burning sulphur or red phosphorous are toxic and corrosive.
The decomposition of self-reactive substances can be initiated by heat, contact with catalytic impurities (acids, bases, heavy metal compounds), friction or impact. Decomposition may result in the emission of toxic gases and vapours. In order to ensure safety during transport, a self-reactive substance may be desensitized using a diluting agent compatible with the substance.
2.5. Class 4.2: Substances liable to spontaneous combustion
Linseed oil (used in paints), copra, oily cotton waste, carbon and white phosphorus are examples of substances which can ignite spontaneously when in contact with air.
These substances are liable to act as a source of ignition for other goods and storage structures. For example, there is a danger of fire if linseed oil spillage are wiped away with rags which then are left to dry in the air. The rags can stay inactive for days before they actually ignite.
2.6. Class 4.3: Substances which in contact with water emit flammable gases
Carbides are among the substances in Class 4.3. An extremely flammable gas, acetylene, is produced by adding water to calcium carbide. When sodium comes into contact with water it gives off hydrogen gas. The reaction is violent and produces enough heat to ignite hydrogen. Hydrogen burns explosively with such a hot flame that metallic material can start to burn. Aluminium and magnesium powders, zinc dust and some metal hydrides are in this class. In addition to the dangers of fire and explosion, goods belonging to this class can react with moisture on human skin and cause burns.
2.7. Class 5.1: Oxidizing substances
In this class are substances such as chlorates, chlorites, nitrates, nitrites, chromic acid and concentrated hydrogen peroxide solution.
These goods should be carefully handled and protected from heat or friction. An oxidizing substance has oxygen bound into its structure. This is liberated by heating and can react with other materials or enhance fire.
Many substances in this class are sensitive to impurities. Concentrated hydrogen peroxide solution begins to decompose if a few rust flakes happen to fall into the container. The reaction starts slowly but accelerates with time. It gives off oxygen which corrodes metallic materials. This can be devastating in transport by rail.
The decomposition of oxidizing goods can also involve liberation of toxic or corrosive gases, such as nitrogen oxides, which can be recognized from their deep brown to yellow brown colour.
2.7.1. Class 5.2: Organic peroxides
This class covers peroxides of organic compounds. They should never be transported or stored with combustible goods. Special recommendations and provisions apply to some of the peroxides because of their high reactivity. In addition to the hazards of explosive decomposition and fire, they are sensitive to impact or friction. Many peroxides are toxic and some of them can provoke allergic response and damage the eyes.
2.8. Class 6.1: Toxic substances
Substances in this class are liable either to cause death or serious injury, or to be harmful when swallowed, inhaled or by skin contact. Toxic substances can be gases, solids or liquids. Toxic gases are listed into the Class 2.3.
Examples of substances in this class are cyanides, arsenic compounds, mercury and lead compounds, nicotine, toluidines, chloroform, aniline and organotin compounds.
In order to compare the various risks involved, LD50 and LC50 are used to assess toxic properties. LD50 means the dose, at which half of the animals exposed (in test laboratory) to the poison die, and LC50 means the concentration which kills 50% of the tested animals after being exposed to the substance.
There are agreed limits for the levels of toxicity measured in animal tests, with reference to the route of exposure. The packing group depends on the amount of the chemical and on the different degrees of health hazard that the chemical poses.
Detailed advice about materials and ways of packing can be found in the United Nations Recommendations on the Transport of Dangerous Goods and in national regulations.
Packing group |
Oral toxicity LD50 (mg/kg) |
Dermal toxicity LD50 (mg/kg) |
Inhalation toxicity by dusts and mists LC50 (mg/litre) |
I |
to 5 |
to 40 |
to 0.5 |
II |
5-50 |
40-200 |
0.5-2 |
III |
Solids 50-200; Liquids 50-500 |
200-1000 |
2-10 |
2.9. Class 8: Corrosive substances
The corrosive substances form a large class. It can be subdivided into acids, bases and other materials.
Examples of acids include hydrochloric acid, sulphuric acid and acetic anhydride. Sodium hydroxide, potassium hydroxide, sodium carbonate and sodium metasilicate are bases or alkalis. Other corrosive substances include antimony pentachloride (textile impregnation), titanium tetrachloride, aluminium chloride and hypochlorites.
The health hazard varies from corrosive to irritating depending on the type and concentration of the active substance.
The corrosiveness of the substance is determined by its pH which measures the level of acidity or alkalinity.
pH |
|
14 13 12 11 10 9 8 7 6 5 4 3 2 1 |
very alkaline (caustic soda)
neutral (water)
very acidic (nitric acid) |
The pH value can often be found on the label or in the documents following the transported substance.
Some countries require that solutions must be classified corrosive, when the pH value is less than 1.5 or greater than 11.5.
The pH of some common substances in dilute water solutions is given below:
| Concentration | Substance | Smell | pH | Effect on skin |
| 1% | Hydrochloric acid | sharp | ~0.6 | Slight feeling |
| 1% | Acetic acid | typical | ~2.8 | None |
| 1% | Sodium hydroxide (caustic soda) | none | ~13.4 | Strong |
| 1% | Ammonia | sharp | ~11.4 | Irritating |
Acids and alkalis are normally transported at very high concentrations, e.g. 90-95% sulphuric acid, 65% nitric acid, 30% hydrochloric acid, 50% sodium hydroxide and 50% phosphoric acid. At these concentrations the pH value is not important, the substances are simply very corrosive.
The substances in this class can attack and corrode many materials, for example, clothe, paper and several metals. Decomposition often produces heat and gases, and in some cases extremely flammable hydrogen gas. The choose of a packing material and loading should carefully planned, because it can take some time before the consequences of corrosive effects are visible. Accidental mixing of different corrosive materials can in some cases lead to violent reactions, which may give off large amounts of gases.
In the case of strong alkalis there is a latent period before a feeling of burning on the skin is experienced. By then the damage is already done. Skin contact with strong acids produces an immediate feeling. Both types of corrosive substances can cause serious skin and eye damages.
Corrosive substances can also present other hazards. For example, benzyl chloride is both toxic and corrosive, diethylamine and butylamine are both corrosive and flammable.
2.10. Class 9: Miscellaneous dangerous substances
These are substances and articles which during transport present a danger not covered by other classes.
Magnetic materials are classified in this category (magnetism may affect the navigation systems of airplanes). PCBs (polychlorinated biphenyls) are placed in Class 9 because they may damage the environment. Dry ice (solid carbon dioxide) can evaporate, producing asphyxiant fumes, and displace oxygen in the air in confined places such as cargo holds in ships and storage cellars. Asbestos can damage the lungs. The effect on health is not immediate; the damage appears after many years. Therefore asbestos is not placed in Class 6.1 but in Class 9.
In this class belong liquids which are transported at temperatures exceeding the boiling point of water; and hot solids.